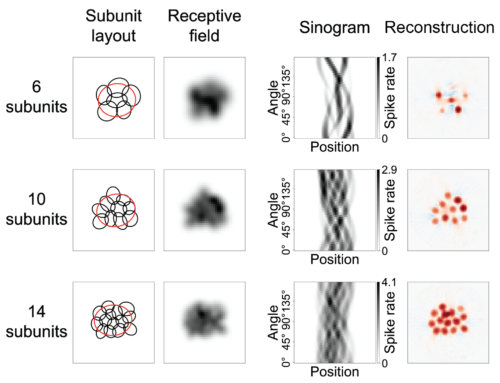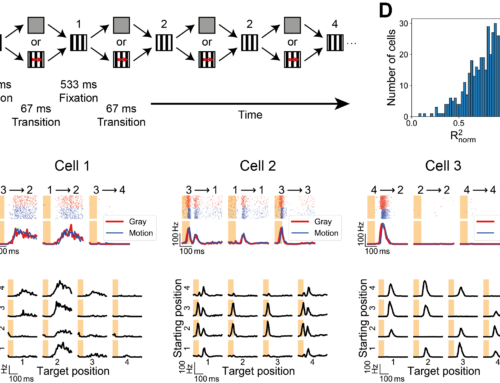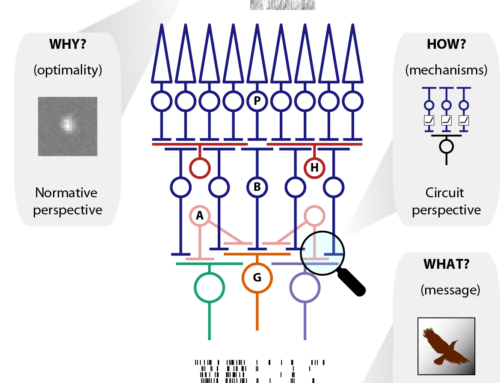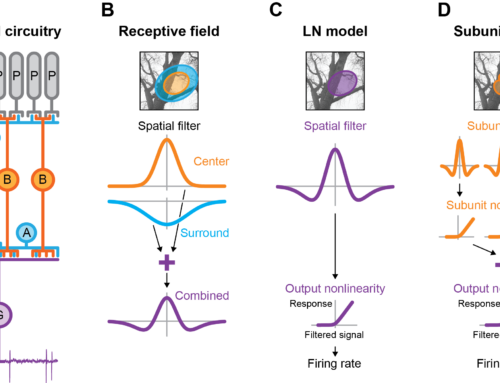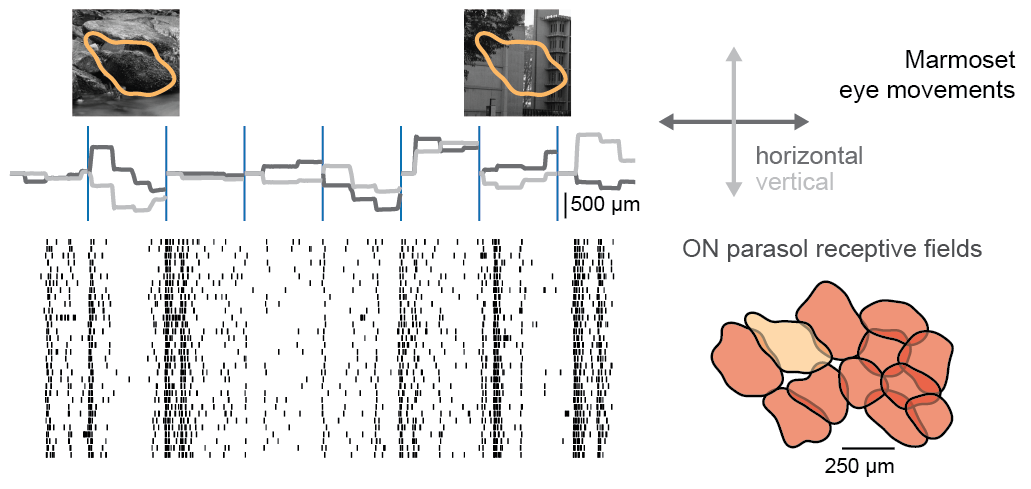
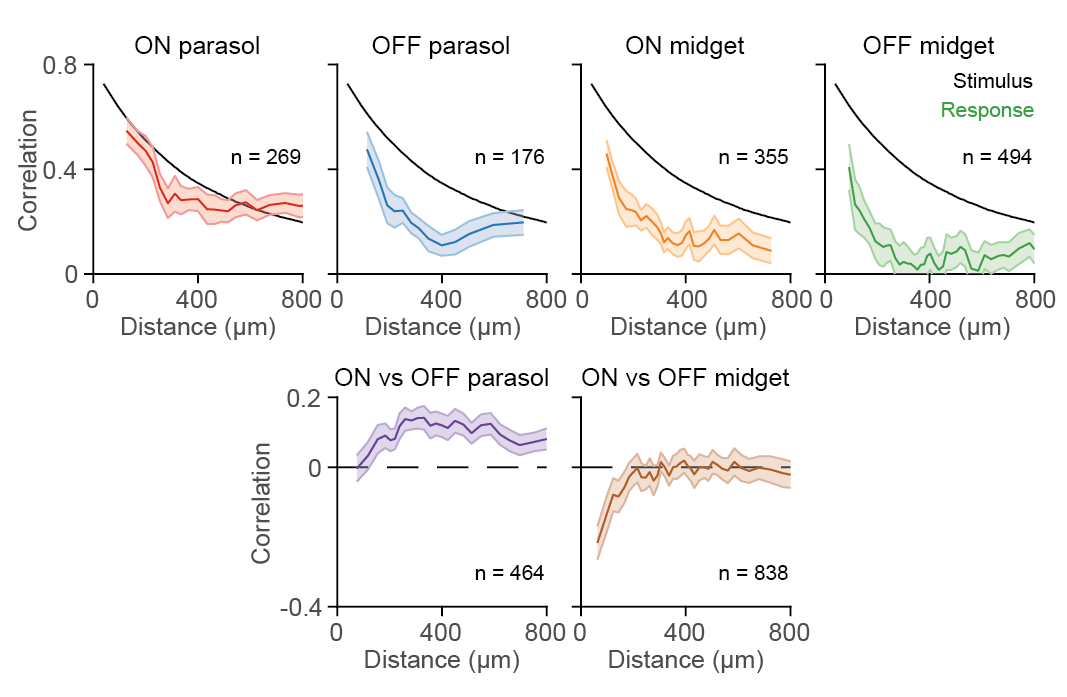
Nonlinear receptive fields evoke redundant retinal coding of natural scenes.
The role of the vertebrate retina in early vision is generally described by the efficient coding hypothesis, which predicts that the retina reduces the redundancy inherent in natural scenes by discarding spatiotemporal correlations while preserving stimulus information. It is unclear, however, whether the predicted decorrelation and redundancy reduction in the activity of ganglion cells, the retina’s output neurons, hold under gaze shifts, which dominate the dynamics of the natural visual input. We show here that species-specific gaze patterns in natural stimuli can drive correlated spiking responses both in and across distinct types of ganglion cells in marmoset as well as mouse retina. These concerted responses disrupt redundancy reduction to signal fixation periods with locally high spatial contrast. Model-based analyses of ganglion cell responses to natural stimuli show that the observed response correlations follow from nonlinear pooling of ganglion cell inputs. Our results indicate cell-type-specific deviations from efficient coding in retinal processing of natural gaze shifts.